Nanochemistry
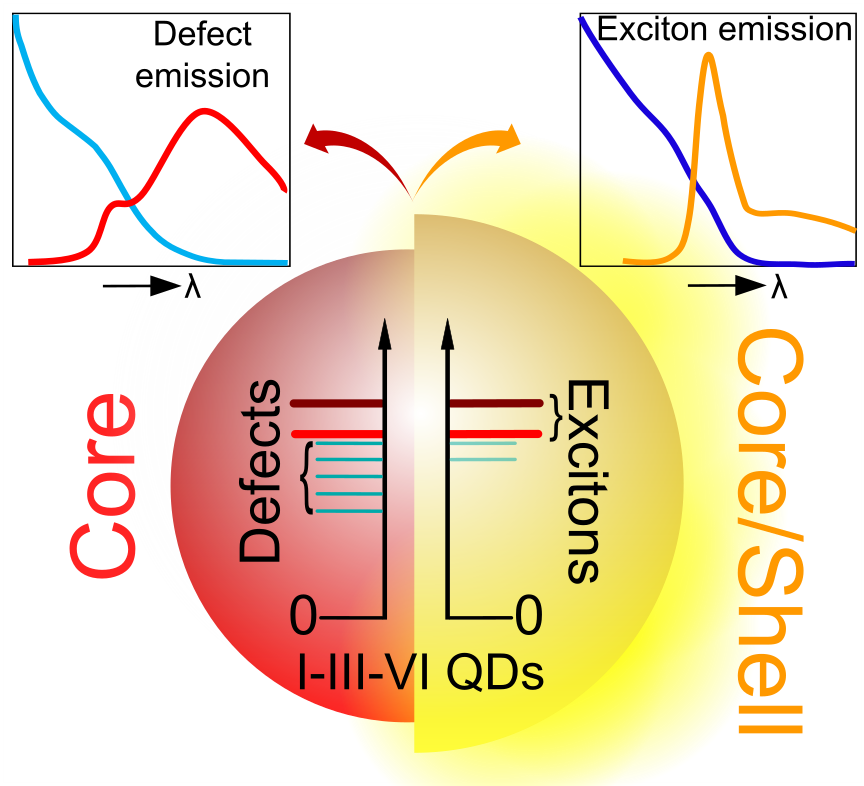
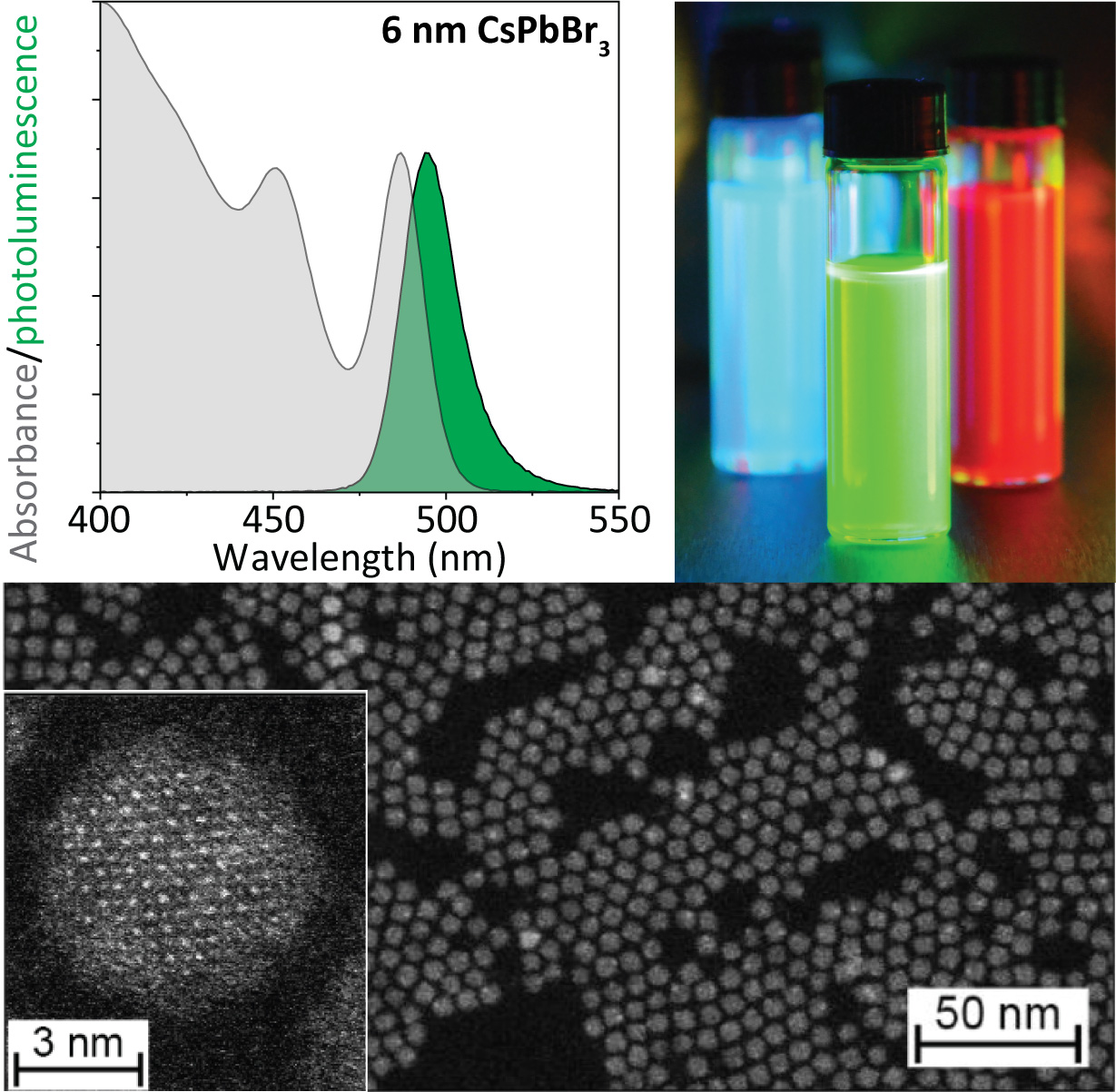
Quantum dots are an exciting group of nm-sized semiconductors whose optical properties can be tuned via their respective sizes. These QDs have attracted the attention due to their light absorption, bright emission of pure colors, control over electronic transport, and a wide tuning of chemical and physical functions across the visible and infrared wavelengths and coherent single photon emission. This makes them promising materials for technologies such as displays and lighting, lasers, sensing, electronics, solar energy conversion, photocatalysis. One of our research interests is to synthesize these quantum dots through colloidal nanochemistry. This allows us to control the size and composition of such tiny semiconductors, and understand their excitonic properties. In doing so, we explore different materials, sizes, shapes and compositions, as well as develop new synthetic methods resulting in extremely monodisperse perovskite quantum dots with tailored surfaces.
Selected work on this topic:
- Efficient Energy Transfer from Quantum Dots to Closely-Bound Dye Molecules without Spectral Overlap. Angew. Chem. Int. Ed., e202420658 (2024).
- Quantum Dot Metal Salt Interactions Unraveled by the Sphere of Action Model. J. Am. Chem. Soc. 2023, 145, 14395–14403 (2023)
- Controlling the nucleation and growth kinetics of lead halide perovskite quantum dots. Science 377, 1406-1412 (2022)
- Spheroidal Cesium Lead Chloride–Bromide Quantum Dots and a Fast Determination of Their Size and Halide Content. Nano Lett. 22, 8168–8173 (2022)